Meet your new car batteries: Water, carbon dioxide, and cobalt
09/09/2019 / By Melissa Smith

Scientists at the University of Massachusetts Lowell developed a safer and more efficient way to power electric cars. The scientists said that this method will allow electric vehicles of all sizes to run longer with zero emissions.
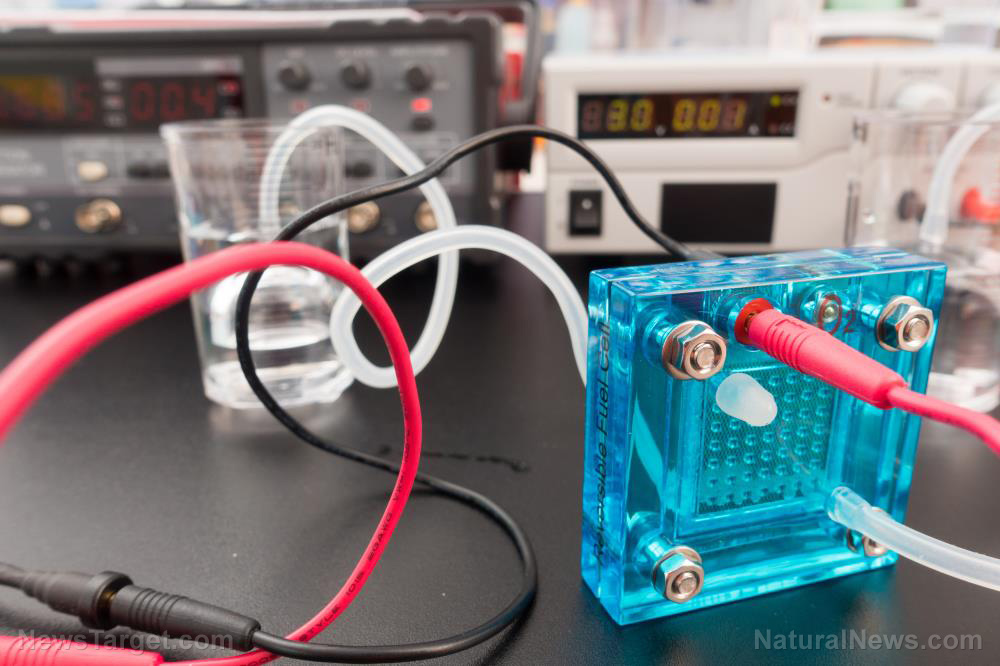
Using water, carbon dioxide, and cobalt, this innovation can produce hydrogen gas on-demand at a relatively low temperature and pressure. Hydrogen is not an energy source, but an energy carrier. This is because it takes a great deal of energy to extract it from water.
In the new method, once the hydrogen is produced, it goes to a fuel cell and is mixed with oxygen from the atmosphere. From there, the hydrogen can be used to generate electricity to power the vehicle’s motor, headlights, and rechargeable battery.
“This process doesn’t store any hydrogen gas, so it’s safe and poses no transportation issues, greatly minimizing the possibility of a fire or explosion,” explained Prof. David Ryan, one of the developers.
The model developed by Ryan and his team is different from the current technology available. Most electric vehicles today, which rely on batteries that must be charged, have limitations such as storage capacity, the time needed to recharge, and cost. As a result, this is practical only for small cars and not for larger vehicles like trucks and buses.
The hydrogen generated in the new method is more than 95 percent pure, making it an even greater way to power vehicles. When hydrogen is burned, it only produces water and no carbon dioxide. But you don’t have to burn hydrogen to produce electricity. Hydrogen is useful as a compact energy source in fuel cells and batteries.
“Hydrogen can be used in fuel cells, in which it combines with oxygen from the air to produce electricity at up to 85 percent efficiency,” said Ryan.
The developers of the technology also suggested that their method would solve the problem of fossil fuel use. However, because hydrogen is not mined or pumped out of the ground like fossil fuels, it must be produced – and current methods of doing that are costly and inefficient.
“This, coupled with the lack of needed infrastructure, has hampered the transition from a petroleum to a hydrogen economy,” said Ryan.
Ryan and his team hope that the catalytic hydrogen technology they have created would help solve all these challenges. They already have a provisional patent on the method and are waiting for a full patent on the technology. In addition to the support from UMass Lowell, the team also received funds from the Massachusetts Clean Energy Center to help take the innovation from the lab to the marketplace. (Related: Breakthrough on-demand hydrogen fuel generator may make hydrogen cars safe and practical.)
Other methods hydrogen fuel is produced
Hydrogen fuel can be produced in several ways, but the most common ones today are natural gas reforming – which is a thermal process – and electrolysis. Thermal processes for hydrogen production involve a high-temperature process called steam reforming. In this method, steam reacts with a hydrocarbon fuel, such as natural gas, diesel, renewable liquid fuels, or gasified biomass, to produce hydrogen.
On the other hand, in electrolysis, water is separated into oxygen and hydrogen. The process takes place in an electrolyzer, which serves much like a fuel cell in reverse. It creates hydrogen from water molecules instead of using the energy of a hydrogen molecule.
Read NewEnergyReport.com for more breakthroughs in clean energy.
Sources include:
Tagged Under: batteries, biotechnology, breakthrough, car batteries, carbon dioxide, Chemistry, cobalt, discoveries, electric cars, electric vehicles, energy, future cars, future science, future tech, goodtech, green energy, hydrogen fuel, hydrogen gas, innovation, research, science and technology, water
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 SOLARPANELS.NEWS
All content posted on this site is protected under Free Speech. SolarPanels.news is not responsible for content written by contributing authors. The information on this site is provided for educational and entertainment purposes only. It is not intended as a substitute for professional advice of any kind. SolarPanels.news assumes no responsibility for the use or misuse of this material. All trademarks, registered trademarks and service marks mentioned on this site are the property of their respective owners.